Extremely strong and heat-resistant
ETH researchers have produced a thin film and extremely fine pillars from a new class of alloys made of multiple finely distributed elements. The material is resistant to extreme pressures and temperatures.
For more than 4,000 years, humans have been making metal alloys in order to obtain materials with desirable properties. Traditionally, these alloys consist of a main metal with smaller quantities of one or a few other elements combined during a smelting process. Bronze, for example, is largely made up of copper, with a smaller proportion of tin, and is significantly stronger than either pure copper or pure tin.
In high-entropy alloys, however, the composition is different. This new class of alloys has been high on the agenda for materials scientists for the past few years, as it offers high strength combined with temperature- and corrosion-resistance. High-entropy alloys typically consist of four or five metallic elements. Researchers led by Ralph Spolenak, Professor of Nanometallurgy, have now used a high-entropy alloy to create a film just 3 micrometres thick, into which they milled a structure consisting of pillars with a diameter ranging from 100 nanometres to 1 micrometre. The alloy contains equal proportions of the elements niobium, molybdenum, tantalum and tungsten.
Modified properties due to fine structure
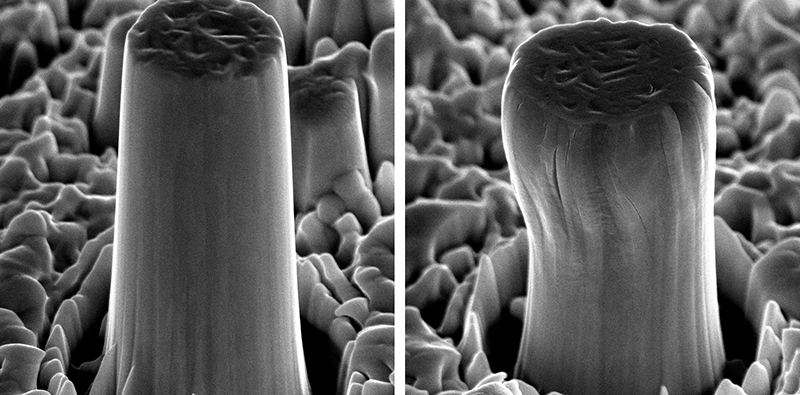
As they found, these “micropillars” made of the high-entropy alloy have very special properties: they are 10-times stronger than a block made of the same material. Furthermore, the pillars can be compressed by up to around a third of their length under high pressures without become brittle or cracking – scientists refer to this deformability as ductility. And, in the end, the material also exhibits enormous temperature-resistance: it survived three days at 1,100 degrees Celsius with no significant change to its external or internal structure – in stark contrast to pure tungsten, which the scientists also subjected to heat treatment as a control. Following heat treatment, micropillars made of the high-entropy alloy perform significantly better in terms of strength and ductility than those made of pure tungsten. This is despite the fact that the high-entropy alloy’s melting point is significantly lower than that of pure tungsten (around 2,900 versus 3,400 degrees Celsius).
The scientists produced the 3 micrometre film using magnetron sputtering, a coating method often used in the field of microelectronics. This was the first time the technique had been used to produce a high-entropy alloy by atomising the four elements mentioned above and spraying them onto a substrate material. Then, using the focused ion beam (FIB) technique, the scientists cut out microcylinders on the surface of the film.
A material made up of tiny individual crystals
The ETH researchers’ material is remarkable not only for its extremely intricate pillar structure but also for its internal crystal structure. Like most crystalline bodies, this material also consists of a large number of small individual crystals. The special feature of the alloy is that these individual crystals are tiny – in scientific terms, this is referred to as a nanocrystalline material. “Although nanocrystalline materials have many desirable properties, they often also bring disadvantages,” explains Yu Zou, a doctoral student in Spolenak’s group and first author of the study, which has now been published in the journal Nature Communications. “For example, these materials are usually not temperature-resistant, as heating causes the individual crystals to expand and therefore changes the properties of the material.”
According to the scientists, the high-entropy alloy’s ability to withstand extreme temperatures may be related to the relatively disordered atomic distribution of the elements inside the material. In particular, the researchers suspect that the disorder at the internal boundary surfaces of individual crystals in high-entropy alloys means the crystals tend to grow less than in other materials when heated. Whether this theory is accurate is something the scientists wish to investigate in another research project, in which they will scrutinise the atomic distribution of elements within the material.
Zou says the new material will be of interest above all in high-pressure and high-temperature applications, for example for building sensors that are required to operate in extreme conditions of this kind.
Literature reference
Zou Y, Ma H, Spolenak R: Ultrastrong ductile and stable high-entropy alloys at small scales. Nature Communications, 10 July 2015, doi: external page10.1038/ncomms8748call_made