Marine bacterium raises hopes
Chemists demonstrate how a newly discovered class of substance from a marine bacterium interferes with the metabolism of plants of pathogens. The substances offer a glimmer of hope in the fight against weeds and pathogens, which keep growing increasingly resistant to current treatment methods.
For a long time, humans could consider themselves safe: medication and antibiotics helped against life-threatening diseases such as malaria or tuberculosis; herbicides kept the undesirable competitors of crops at bay and helped increase yields. Now these weapons we humans rely on are rapidly becoming ineffective as bacteria and weeds have developed a resistance to the methods we use to destroy them. Consequently, researchers are frantically looking for new substances and effect mechanisms to regain control of diseases and weeds.
A research consortium headed by François Diederich, a professor of organic chemistry at ETH Zurich, has now come across a possible solution. In a new study published in the journal Angewandte Chemie, the scientists describe a new group of substances and previously unknown mechanisms of action that could be used to combat both malaria pathogens and weeds.
Terpenes ensure survival
The substances interfere with a particular metabolic process of these organisms that does not occur in mammals. Plants and various single-cell organisms, including the malaria and tuberculosis pathogens, produce terpenes via the so-called non-mevalonate biosynthetic pathway. This substance class includes steroids or carotenoids. “Terpenes are extremely important plant substances, without which they can’t survive,” says Diederich.
This synthetic pathway was discovered and first described in the 1990s. Scientists subsequently found seven enzymes that reconstruct different precursors chemically to form terpenes. These enzymes were recognised as targets for therapeutic interventions: if they can be inhibited with a suitable substance, the synthesis of terpenes breaks down and the organism affected dies.
Well-known substances rediscovered
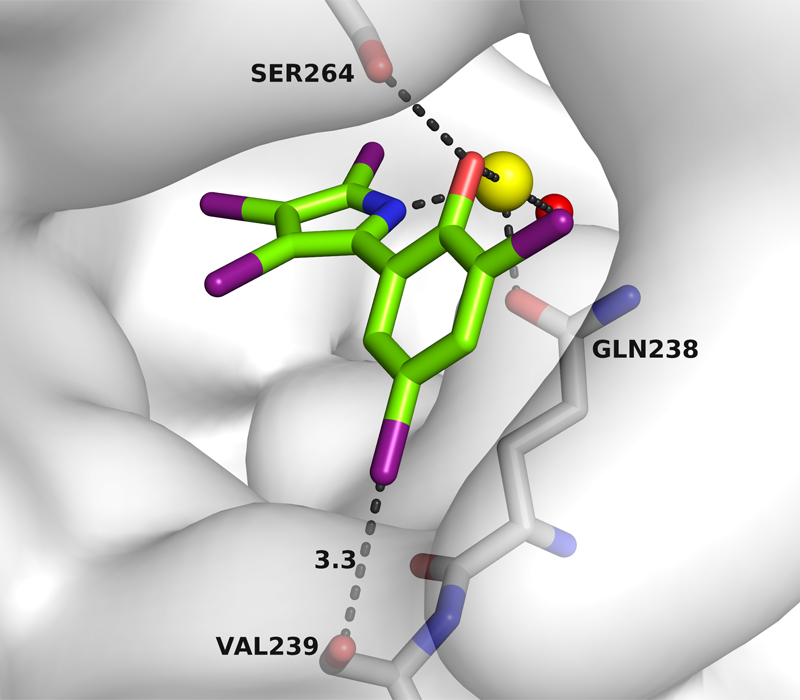
A commercially available pesticide blocks the first enzyme in this chain, an antibiotic the second. And as researchers now reveal, the third enzyme (IspD) can also be inhibited – with pseudilins. The scientists stumbled across these agents thanks to a specific search in the substance library at the chemical company BASF. Pseudilins were already isolated from a marine bacterium of the Pseudomonas species back in the 1960s.
In their study, the researchers now describe how pseudilin derivates affect IspD. These substances are characterised by an unusually high number of halogens such as bromine and chlorine. They accumulate in a pocket-shaped pit on the enzyme surface via halogen bridge bonds and a metal ion complex, as a result of which the enzyme changes its original form so that it is unable to convert any more substrates. This pocket has the advantage that pseudilins of different chemical compositions fit into it, which means the often exclusive lock-and-key principle of the enzyme and substrate is not necessary to bind the substances.
The researchers tested the rediscovered agents on wall cress, a commonly used model plant, and the malaria pathogen Plasmodium vivax. The results caused quite a stir: the pseudilins were effective against both organisms, as different as they are. Mammalian cells, however, do not react as they form terpenes via a different metabolic pathway.
Only ten out of 1900 ideas successful
Diederich is now looking to produce and evaluate other even more effective pseudilin derivates. Their activity would have to be improved by a factor of 100 for them to be of interest to pharmaceutical research. At the same time, he urges people not to get their hopes up too much for the new miracle agents. “According to a study conducted by the University of St. Gallen, of the 1,900 ideas in the product pipeline only fifty actually make it onto the market,” says the ETH-Zurich professor. And only one in five products launched becomes a blockbuster.
In this research project about pseudilins, various partners participated. Besides ETH Zurich, the Tropical Institute at the University of Basel, TU Munich, the universities of Dresden and Hamburg, and the industrial partner BASF were involved. The Pseudlin project is part of a larger programme for structure-based drug design. “We’re talking big science here,” says Diederich. “These days, you can only achieve this in large consortiums, where fifty or sixty researchers join forces.”
Further reading
Kunfermann A et al. Pseudiline: halogenierte, allosterische Inhibitoren des Enzyms IspD aus dem Mevalonat-unabhängigen Biosyntheseweg. Angewandte Chemie 2014, 126, 1-7, DOI: external page10.1002/ange.201309557call_made
