A toolbox for creating new drugs
ETH microbiologists led by Markus Künzler have discovered a remarkable enzyme in a fungus. They now want to use it to develop new drugs.
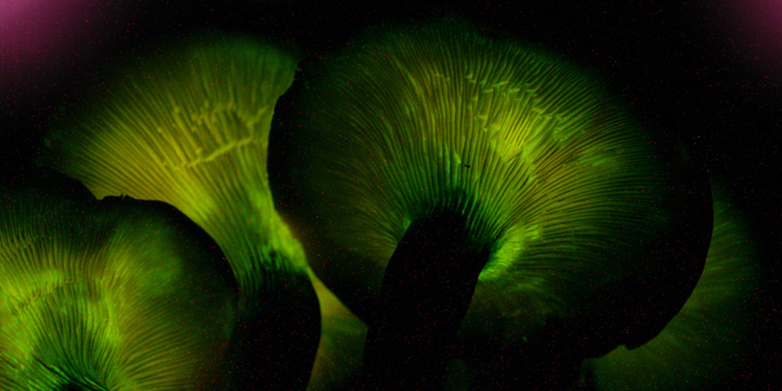
This fungus is full of surprises: the jack-o’-lantern mushroom glows in the dark and produces peptides that could be useful for humans. (Image: Noah Siegel, wikicommons, CC BY-SA 3.0)
Fungi appear to offer a truly inexhaustible reservoir of new substances. One such fungus is the jack-o’-lantern mushroom (Omphalotus olearius), which is found throughout the Mediterranean region and has a fruiting body that glows in the dark.
But it’s not this special effect that has medical researchers interested; it’s an enzyme recently discovered by ETH researchers led by the microbiologist Markus Künzler.
This enzyme, OphA, forms a key part of a metabolic pathway that keeps pests away from the jack-o’-lantern mushroom. “Fungi protect themselves from predators and competitors using a cocktail of toxins, many of which are proteins or peptides,” says Künzler.
The jack-o’-lantern mushroom uses the OphA enzyme to provide the backbone of one of these peptides with additional methyl groups. Only upon this chemical alteration and subsequent cyclisation does the peptide, omphalotin A, function as a toxin. The mushroom uses it to ward off pests such as roundworms.
A production line that’s difficult to manipulate
Fungal peptides also serve as drugs in medicine. One of the most well-known is cyclosporin A, which has been used in organ transplants, autoimmune diseases and cancer medicine for almost 40 years.
This peptide carries methyl groups on its backbone as omphalotin A. The ring shape and the methyl groups are responsible for cyclosporin A’s advantageous pharmacological characteristics, in particular its oral availability – a factor which is at present a significant obstacle for peptide-based drugs.
Unlike omphalotin A, whose backbone, like that of the majority of proteins and peptides in a cell, is produced by the ribosome, cyclosporin A is built from amino acids by a huge enzyme dedicated to this task. This enzyme functions much like a production line in the automotive industry. “However, it’s difficult to biotechnologically alter this production line to produce variants of cyclosporin A,” says Künzler.
Creation of new ring-shaped peptides
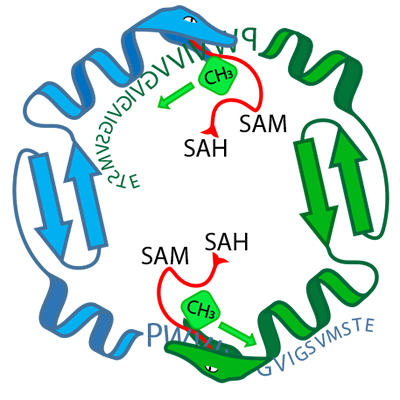
Using the OphA enzyme, however, it might be possible to create new ring-shaped peptides rather than simply variants of omphalotin A. Thanks to the methyl groups attached to them, these new peptides would possess similarly advantageous pharmacological characteristics to those of cyclosporin A.
This possibility is due to the fact that peptides modified by OphA are ribosomally produced and thus can be easily altered via changes to the peptide-encoding genes. In addition, OphA appears to be less selective about the peptides that it can alter chemically. The enzyme is therefore able to attach methyl groups to a wide range of different peptides.
“We can produce biotechnologically different starting peptides and alter them using OphA, which may allow us to create entirely new peptides with pharmacologically advantageous characteristics,” explains Künzler.
Patent applied for
OphA thus enables the production of libraries of ring-shaped peptides with methyl groups as a basis for the development of peptide drugs. These peptide libraries could then be screened for peptides that display a desired biological characteristic, such as the ability to bind to a target protein in cancer therapy.
Due to the attached methyl groups, peptides identified in this way have a high probability of manifesting advantageous pharmacological characteristics. This means that they represent promising lead peptides for the development of respective peptide therapeutics. “This approach offers clear advantages with regard to cost efficiency and probability of success when compared with approaches where methyl groups are only introduced into the peptide afterwards,” says the microbiologist.
ETH Zurich has applied for a patent for the use of OphA and related enzymes to insert methyl groups into ribosomal peptides and for the procedure used to create corresponding peptide libraries. Künzler has received a CTI grant, currently without an industry partner, to provide the proof of principle that the technology works. A spin-off is also under discussion. “However, we need to provide the proof of concept for the technology before we can begin raising funds for a potential spin-off.” The next two years will likely make or break this plan.
Enthusiastic experts
Künzler’s results have been enthusiastically received among experts in the field. Interest in his system was high at a recent conference on peptides in Canada, and a company has also expressed interest in acquiring a licence on the patent. “It is fun to guide fundamental research towards practical applications,” says Künzler.
He believes that fungi have the potential to provide a wide range of naturally occurring agents. There are millions of different species of fungi, and the number of promising natural substances is correspondingly large. However, he remains a realist and acknowledges that only a small fraction of these will be applicable as drugs. Perhaps some of those from his collection of peptides will be among them.
References
van der Velden NS, Kälin N, Helf MJ, Piel J, Freeman MF, Künzler M. Autocatalytic backbone N-methylation in a family of ribosomal peptide natural products. Nature Chemical Biology (2017). doi: external page10.1038/nchembio.2393call_made
