How salt stress changes the membrane
ETH researchers have found an unknown mechanism that helps bacteria withstand salt stress. They incorporate the lipid ubiquinone-8 into the membrane surrounding them. This stabilises the membrane and prevents the cell collapsing.
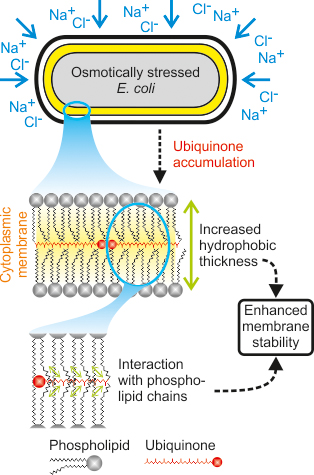
A cell is, in principle, a narrow, constrained space filled with numerous water-soluble substances that swim around in an aqueous medium. If the concentration of water-soluble substances in the medium is far smaller than in the cell, water flows into the cell to balance out the concentration gradient. If, by contrast, there is a higher substance concentration in the medium than in the cell, then the water flows back from it into the environment. In the first instance, the cell could burst, in the second case it shrinks considerably. These situations, which biologists call osmotic stress, impact from time to time all cells, irrespective of whether they are plant cells, bacteria or cells in the human body. Cells are well equipped to cope with these situations, too. Drawing on various strategies known to research, they prevent themselves from bursting or collapsing as a consequence of changes in the medium surrounding them. For instance, they ramp up their metabolism and produce various sugars.
Crosswise to the main direction
The two ETH researchers, Uwe Sauer and Daniel Sévin, have now found a previously unknown and surprising way in which the microorganisms, Coli bacteria (Escherichia coli), withstand salt stress – i.e. a high salt content in the environment that leads to high osmotic pressure. The corresponding work has just been published in Nature Chemical Biology. In a high salt environment the bacteria produce large volumes of a special lipid, ubiquinone-8 (Q8). This lipid is then incorporated to strengthen the bacterium’s membranes.
Membranes consist of a lipid bilayer. This, in turn, consists of a phosphate headgroup to which long carbon chains are bound. The headgroup is “water-friendly” whereas the chains are “water-shy”. Hence, they turn towards each other, excluding any contact with aqueous solutions, and the headgroups more or less form the outer demarcation to the surrounding medium and to the inner cell. The two ETH researchers were able to demonstrate that Q8 is incorporated into the membrane and probably comes to rest like a cross-brace between the rows of carbon chains.
One percent is already enough
The higher the salt content of the medium rises, the more lipids the membrane contains. At an extremely high salt level of 500 millimolars they amount to one percent. By contrast, in an aqueous solution with a normal salt content (50 millimolars) the Q8 proportion is so small that it can no longer be measured. “It seems that this one percent already suffices in order to make the membrane more rigid”, says Uwe Sauer, “so there is no need for a complete membrane rearrangement.”
The researchers examined whether Q8 or the human variant Q10 helps to strengthen the membrane using, amongst other things, artificially engineered vesicles, small bubbles which like cells are surrounded by a lipid bilayer membrane. If these vesicles contained a Q10 proportion of three to five percent they were more stable than ones containing no Q10.
Q8 is not an unknown molecule to researchers. It plays a role as a transport vehicle for electrons in cellular respiration. However, what was not known up to now was that it is formed in larger numbers in the cell as protection against salt stress. Sauer and Sévin have since observed the synthesis of a large volume of Q8 in other bacteria and yeast cells, too.
Chance finding during routine check
Sauer and Sévin came upon Q8 as another mechanism used by the cell to withstand salt stress almost by chance. Their original intention had been to use a specific mass spectrometry method to measure metabolites in Coli bacteria which are produced as a response to stress. During this work they came upon the highly regulated Q8 and the effect described above.
The consequences of this observation are still unclear. The effect could be exploited, at best, in biotechnology. It could be helpful in promoting the formation of Q8 in bacterial cultures in order to achieve higher stress resistance in industrial reactors. There were also human diseases like nephrotic syndrome in which the Q10 concentration is reduced. In mice it was shown that a mutation in the Q10 biosynthesis pathway leads to Parkinson-like symptoms. “In the past they were generally linked to cellular respiration problems. Our discovery, however, indicates that this could be linked to membrane stability”, stresses the system biologist.
Further reading
Sévin DC & Sauer U. Ubiquinone accumulation improves osmotic-stress tolerance in Escherichia coli. Nature Chemical Biology, published online 9th February 2014. DOI: external page10.1038/nchembio.1437call_made
