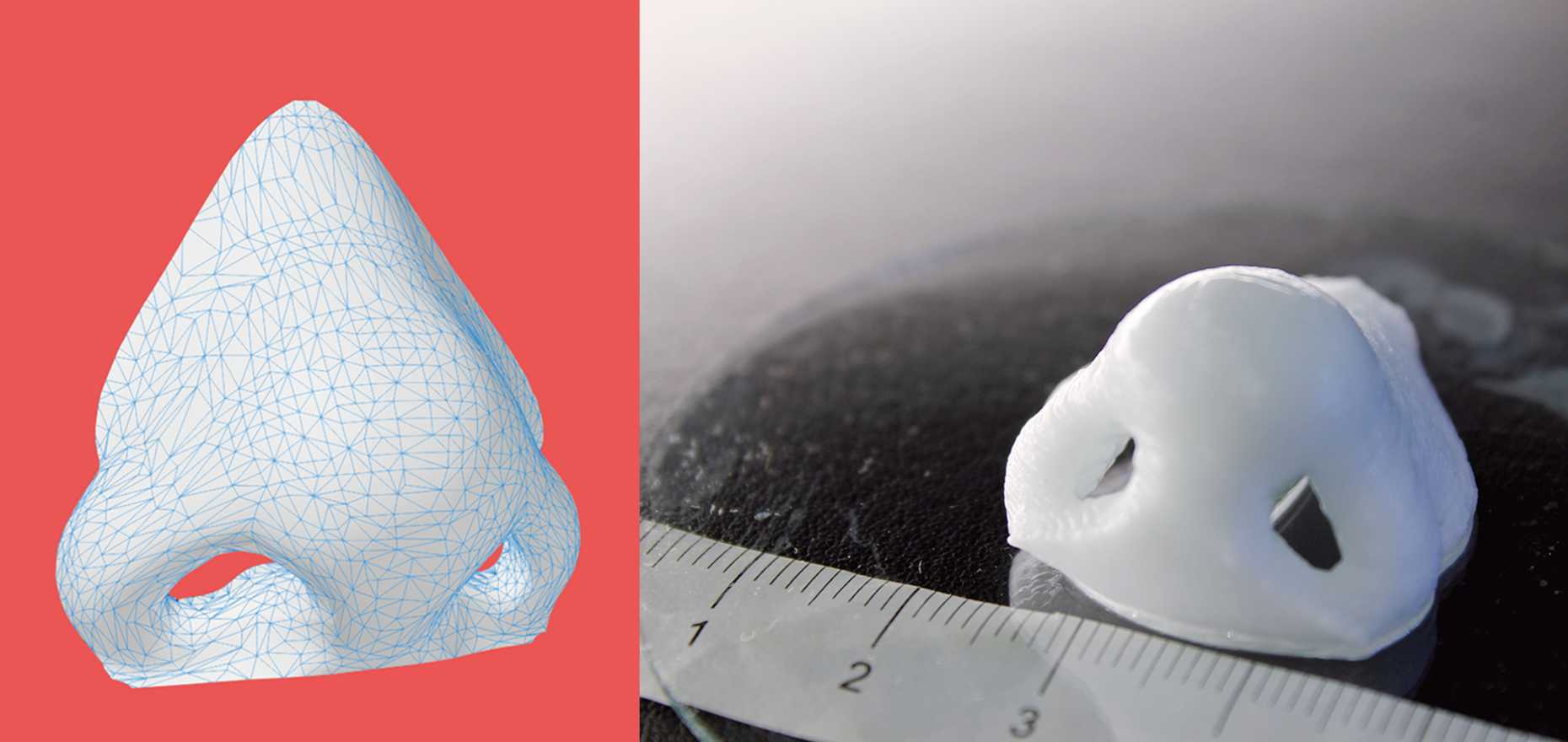
Nose made by bioprinters
3D printers are opening up new opportunities in medicine too. A group of researchers in a team led by Marcy Zenobi-Wong is printing cartilage transplants using the body’s own cells. They are personalised and grow with the patient.
Bioprinting, 3D printing with cellular materials, is currently well on the way to becoming the next big thing in personalised medicine. In the laboratory of the Cartilage Engineering and Regeneration group at the Department of Health Sciences and Technology Matti Kesti presents the latest results of their research: a bowl filled with nutrient solution contains murky white cartilage forming a nose and miniature ear. The doctoral student created both of these from a mix of biopolymers and living cartilage cells using the laboratory’s own bioprinter, a 3D printer for biological materials. This remarkable printer is as big as a laboratory hood and at first glance resembles a protected extraction device in the laboratory. The heart of the system is a wheel with eight syringes that can all be filled with a different suspension. Using a computer outside the lockable printer, the pistons of the syringes are controlled using digital data from a three-dimensional model. The suspension is then ejected from the syringe nozzle at the highest level of precision and a random structure is created on a platform below, which whizzes to and from at rapid speed, using the layering method. This method can be used to create structures such as a joint cartilage or a nose cartilage, which the bioprinter takes just 16 minutes to create.
Kesti outlines how this method may revolutionise reconstructive surgery in the future: A serious car accident results in a passenger’s nose being shattered. It is possible to reconstruct this as a 3D model on the computer. At the same time, a biopsy is performed on the patient and cartilage cells removed from his or her own body, for example from the knee, finger, ear or splinters of the shattered nose. The cells are spawned in the laboratory and mixed with a biopolymer. From this toothpaste-like suspension, a nose cartilage transplant is created using the bioprinter, which is implanted in the patient during surgery. In this process, the biopolymer is used merely as a form of shaping mould; it is subsequently broken down by the body’s own cartilage cells. After a couple of months, it is impossible to distinguish between the transplant and the body’s own nose cartilage. This procedure has significant benefits compared to traditional implants, for instance those made from silicone: the risk of the body rejecting the implant is a lot lower. A particularly crucial factor for young patients is that the cellular implant grows together with the patient, because it is controlled by the patient’s internal growth engine, as is the case for other body parts.
Stringent requirements for bioink
There are many reasons why cell printing is finding its way into medicine just now: “3D printing has been around for nearly 20 years. The fact that it is only just being discovered for surgical purposes is, in particular, down to the lack of bioinks,” explains Marcy Zenobi-Wong, professor and head of Kesti’s research group. Commercial cellular printer cartridges do not yet exist due to the extreme requirements placed on the materials used in transplants. All materials used in clinical procedures are subject to strict international and national guidelines and need to undergo years of testing before being used in hospitals, a process that can cost millions. For that reason, Zenobi-Wong and her group of researchers use biopolymers, with which they are already familiar given their widespread use in everyday hospital procedures. These biopolymers include alginic acid, which is extracted from seaweed and is easily tolerated by the human body, or chondroitin sulfate, a macromolecule generated by the human body, which is responsible for the resistance in cartilage tissue.
Such biopolymers are prepared for the bioprinting process by adding human cells (or for laboratory use, animal cells) and processed to create a hydrogel comprising up to 90 per cent water. The liquid properties of this bioink need to be just right for the gel not to block the syringe cannulas, but the gel must also be sufficiently viscous so that the structure of the object to be built actually holds. If the gel were too li-quid, the layers would melt during printing. The gelatinisation properties must also be taken into account, because for the gel to become a solid structure that doctors can use, it must have a fixed form. This is done by polymerising the hydrogel, which is initiated by light, temperature, a change in pH value or by adding ions. “We have very little room for manoeuvre here,” explains Zenobi-Wong, “because we need to be careful at all times that the cells are not damaged during the printing process.” She therefore devotes a large part of her research to looking for suitable biopolymers and forms of polymerisation that do not damage the cells.
Third dimension as a pointer
One of the first applications for printed cartilage transplants could be in treating injuries to knee and ankle joints. Cartilage transplants are already performed on younger patients with sports injuries, as part of which the patient’s own cartilage cells are cultivated on hydrogel bands in the laboratory. A suitable section of this band is chosen and then sewn into the injured area. Although this is a good approach, it is not ideal because two-dimensional cell growth in the laboratory lacks key spatial information for the future functioning of the patient’s joint. The cells therefore form scar-like tissue instead of cartilage mass. As cells and their supporting structure – the so-called cellular matrix – are printed in the same step using a bioprinter, their future use is clear from the very outset. This allows the cells to retain their original features and reproduce new cartilage from the patient’s body.
The first transplants involving a bioprinter are to be tested in sheep and goats as early as this year. Such tests in large animals must be carried out before clinical trials can be performed on humans, which in turn, paves the way for everyday use in hospitals. “Whether we will see bioprinters in hospitals in the future, however, is less of a technical question; instead, it depends on whether the technology will be accepted by doctors, patients and insurers,” says Zenobi-Wong. Her group of researchers is therefore already collaborating closely with health professionals from the Schulthess Clinic.
Hearts out of the printer?
Since the first international workshop on bioprinting was held in 2004, this area of research has grown continuously. More than 80 research groups are currently working on potential applications in clinical procedures and the first commercial providers of “printed” cell structures for clinical trials have already entered the US market, backed by a healthy amount of venture capital. Does this mean that hearts and kidneys will soon follow the first printed and implanted cartilages, as some are forecasting? Zenobi-Wong is not so sure: “While there’s great deal of hype around bioprinting at the moment, our research is a long way from offering things that are already being promised today.” Producing cartilage is relatively simple compared to body organs, which need to be supplied with blood and large quantities of oxygen immediately. When it comes to the heart, lungs or kidneys, hundreds of capillaries would need to be printed to supply the organ, and this needs to be done to a degree of precision and using materials that will likely not be possible for a long time to come. In contrast to cartilage, various cells need to communicate with one another in such organs in order to perform a whole series of different functions. “Our expertise is in cartilage, probably the easiest bodily tissue for bioprinting,” says Zenobi-Wong, “but even today we know that this is anything but easy to print.”